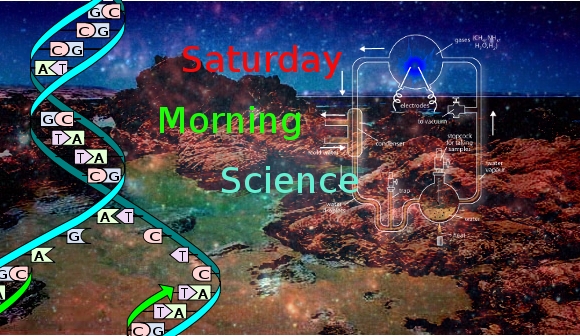
Saturday Morning Science 008
“…a warm little pond”
That simple image of a lukewarm puddle comes from a letter that Charles Darwin wrote to botanist Joseph Hooker in 1871, wherein he speculated on the possible, purely chemical origins of life.
It is often said that all the conditions for the first production of a living organism are present, which could ever have been present. But if (and Oh! what a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, etc., present, that a protein compound was chemically formed ready to undergo still more complex changes, at the present day such matter would be instantly devoured or absorbed, which would not have been the case before living creatures were formed. It is mere rubbish thinking at present of the origin of life; one might as well think of the origin of matter.
Rubbish or not it didn’t stop biochemists from running experiments to recreate the initial beginnings of terrestrial life. So far no one has done it but there have been some interesting starts.
 The Miller-Urey Experiments– In 1952, two researchers at the University of Chicago, Stanley Miller and Harold Urey first synthesised simple organic compounds from inorganic matter. Simulating the current ideas as to the atmospheric composition of the primitive Earth, they subjecting the gas/water vapour mixture to electricity and produced a residue of amino acids. Not living matter but one of it’s most basic ingredients and very necessary for the formation of proteins. Apparently they may have produced more than they reported.
The Miller-Urey Experiments– In 1952, two researchers at the University of Chicago, Stanley Miller and Harold Urey first synthesised simple organic compounds from inorganic matter. Simulating the current ideas as to the atmospheric composition of the primitive Earth, they subjecting the gas/water vapour mixture to electricity and produced a residue of amino acids. Not living matter but one of it’s most basic ingredients and very necessary for the formation of proteins. Apparently they may have produced more than they reported.
Samples from experiments that Miller ran in 1958 and thought to be lost, turned up in 2007 and have been re-examined using present day techniques. As reported in Science Daily:
Former Scripps undergraduate student Eric Parker, Bada and colleagues report on their reanalysis of the samples in the March 21 issue of Proceedings of the National Academy of Sciences. Miller’s 1958 experiment in which the gas hydrogen sulfide was added to a mix of gases believed to be present in the atmosphere of early Earth resulted in the synthesis of sulfur amino acids as well as other amino acids. The analysis by Bada’s lab using techniques not available to Miller suggests that a diversity of organic compounds existed on early planet Earth to an extent scientists had not previously realized.
“Much to our surprise the yield of amino acids is a lot richer than any experiment (Miller) had ever conducted,” said Bada.
The new findings support the case that volcanoes — a major source of atmospheric hydrogen sulfide today — accompanied by lightning converted simple gases into a wide array of amino acids, which are were in turn available for assembly into early proteins.
Bada also found that the amino acids produced in Miller’s experiment with hydrogen sulfide are similar to those found in meteorites. This supports a widely-held hypothesis that processes such as the ones in the laboratory experiments provide a model of how organic material needed for the origin of life are likely widespread in the universe and thus may provide the extraterrestrial seeds of life elsewhere.
Meanwhile Back at the Reactor: The situation at the Fukushima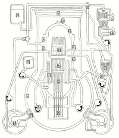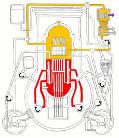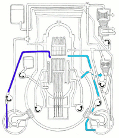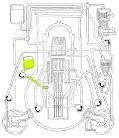 nuclear plant continues. If you’d like to get a handle on just how this works (or doesn’t) the best article I’ve seen is from Mother Jones; Japanese Nuclear Reactor Systems Drawn Like a NYC Subway Map. It’s sounds flip but it’s the best overview that anyone has attempted. Thanks to Abbas Raza and 3 Quarks Daily for this one.
nuclear plant continues. If you’d like to get a handle on just how this works (or doesn’t) the best article I’ve seen is from Mother Jones; Japanese Nuclear Reactor Systems Drawn Like a NYC Subway Map. It’s sounds flip but it’s the best overview that anyone has attempted. Thanks to Abbas Raza and 3 Quarks Daily for this one.